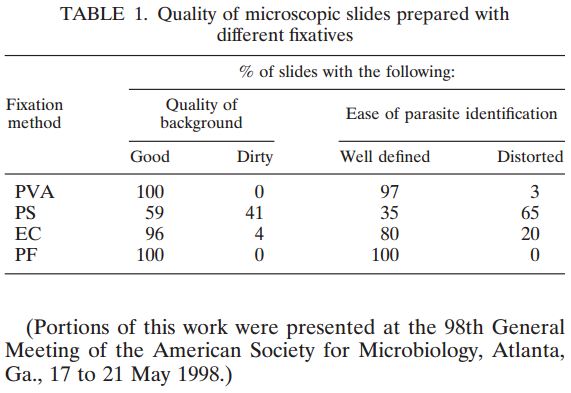
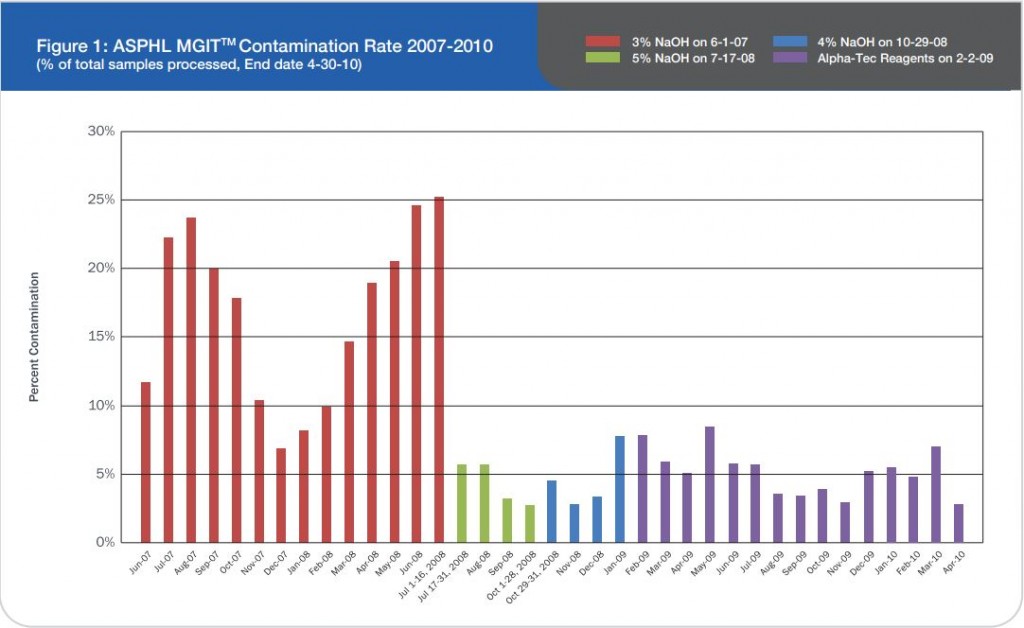
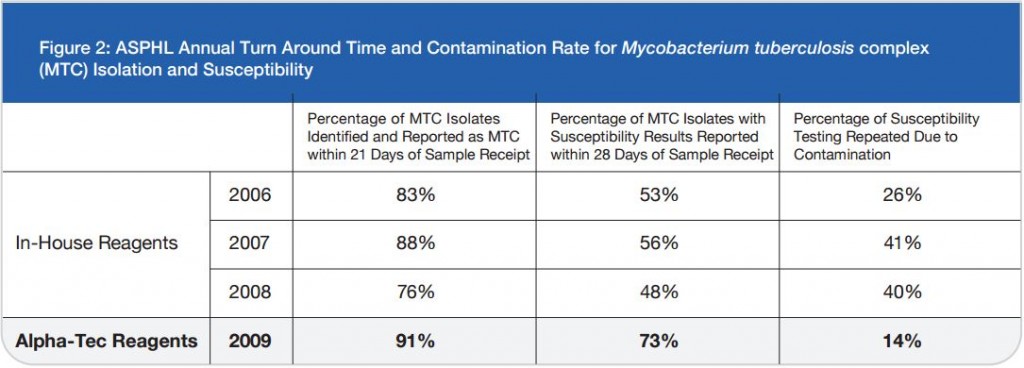
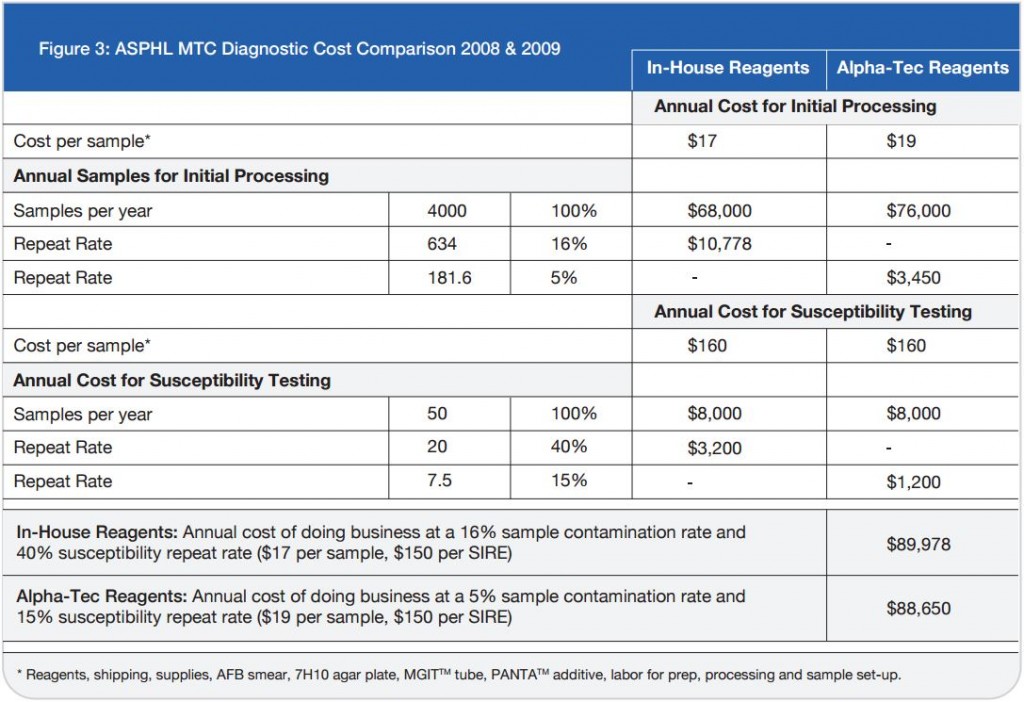
Ģirts Šķenders, Inga Prokopoviča, Lonija Broka — Infectology Center of Latvia, Mycobacteriology Department/Riga, Latvia — Presented at the 2010 European Congress of Clinical Microbiology and Infectious Disease
Abstract and Background
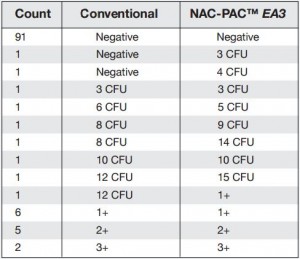
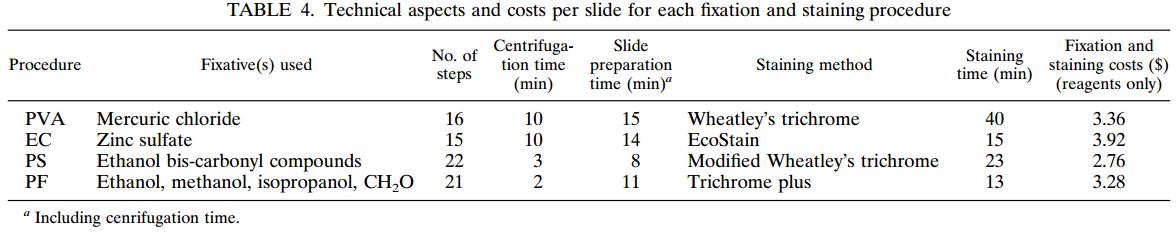
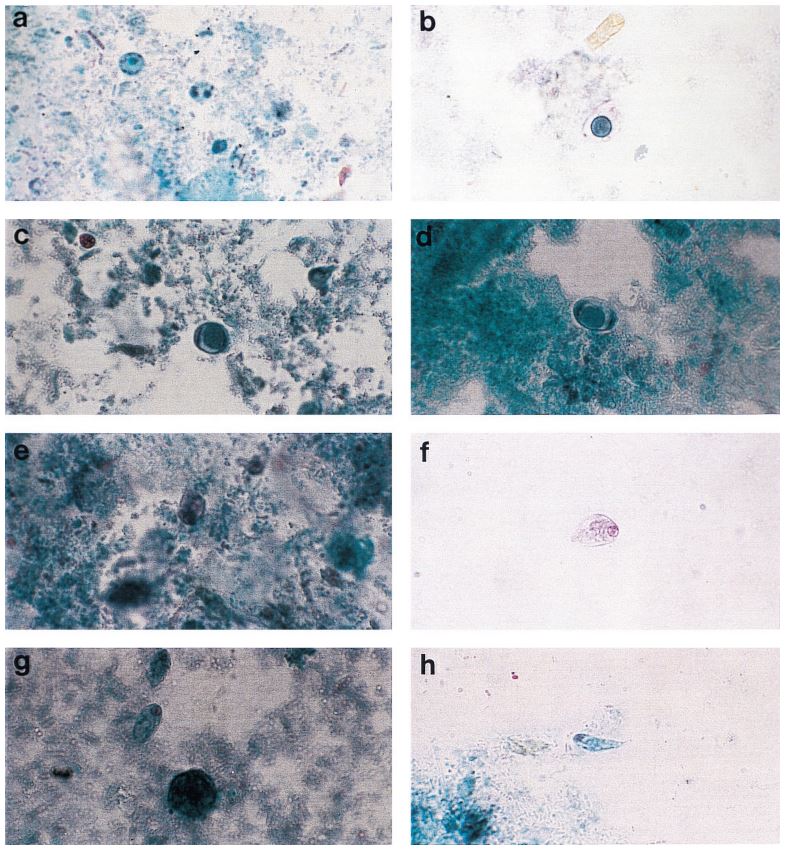
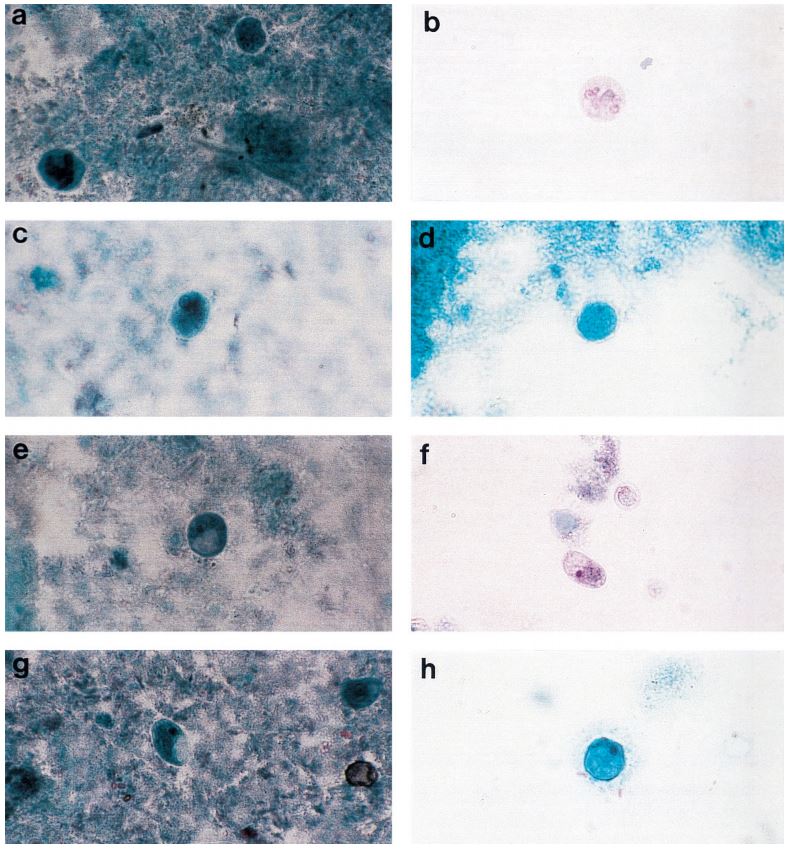
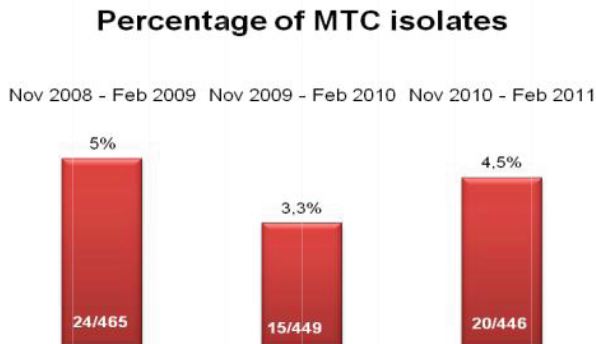
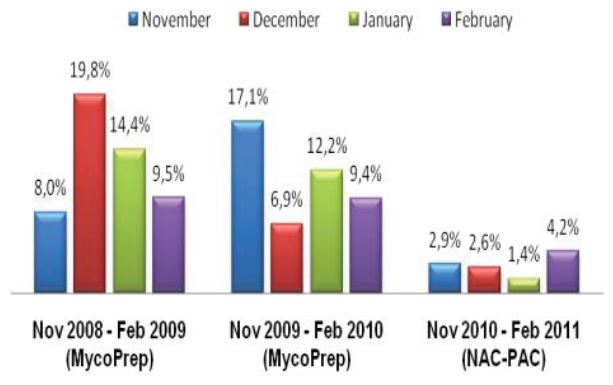
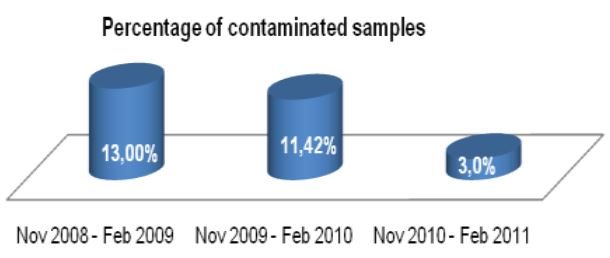
Figure 1: Comprehensive results of
specimen preparation evaluation
Sputum and other lower respiratory specimens are the most common samples used in the diagnosis of tuberculosis and other mycobacteria. These samples are highly mucoid in nature, and since they must pass through the oral-pharyngeal cavity, they are almost invariably contaminated during collection. In order to be useful for diagnostics, sputum and lower respiratory specimens must be liquefied in order to free organisms from mucoid strands, and all contaminating flora must be removed to prevent overgrowth that will mask the presence of mycobacteria in culture. In order to liquefy and decontaminate the sample, laboratories must undertake a rigorous pre-analytical specimen preparation process. While there have been advances in the analytical technologies used to diagnose tuberculosis, the generally accepted pre-analytical specimen preparation process has remained essentially unchanged, and as it is typically performed can significantly reduce the number of viable mycobacteria available for diagnosis. The conventional methodology for specimen preparation exposes a raw lower respiratory sample to a high pH digestion and decontamination reagent in order to remove any contaminant oral-pharyngeal flora as well as breaking down the disulfide bonds which trap bacteria within mucoid strands. After a prescribed exposure period, phosphate buffered saline (PBS) or M/15 phosphate buffer is added to the sample.1
This sample and reagent mixture is then centrifuged to sediment any organisms present and decanted to create the specimen used for all diagnostic protocols. As it is typically performed, this protocol has significant shortcomings; neither M/15 nor PBS can neutralize the NaOH used as a decontaminant and leaves the sample at a pH above 12, elevated enough to significantly reduce the number of viable mycobacteria. After centrifugation, the diagnostic sample is created either via incomplete decantation or using the same PBS or M/15 used as a dilution reagent; this leaves the sample with a highly variable final pH and concentration which can yield erroneous results in liquid culture diagnostics and create improper samples for microscopy. Alpha-Tec® Systems, Inc. offers the NAC-PAC™ EA3, a system of reagents designed to replace those used during the conventional specimen process and provide greater control over sample pH and characteristics. This study aims to determine if an updated method for specimen processing can have a meaningful positive impact upon the final diagnostic outcome in the detection of tuberculosis and other mycobacteria.
Materials and Methods
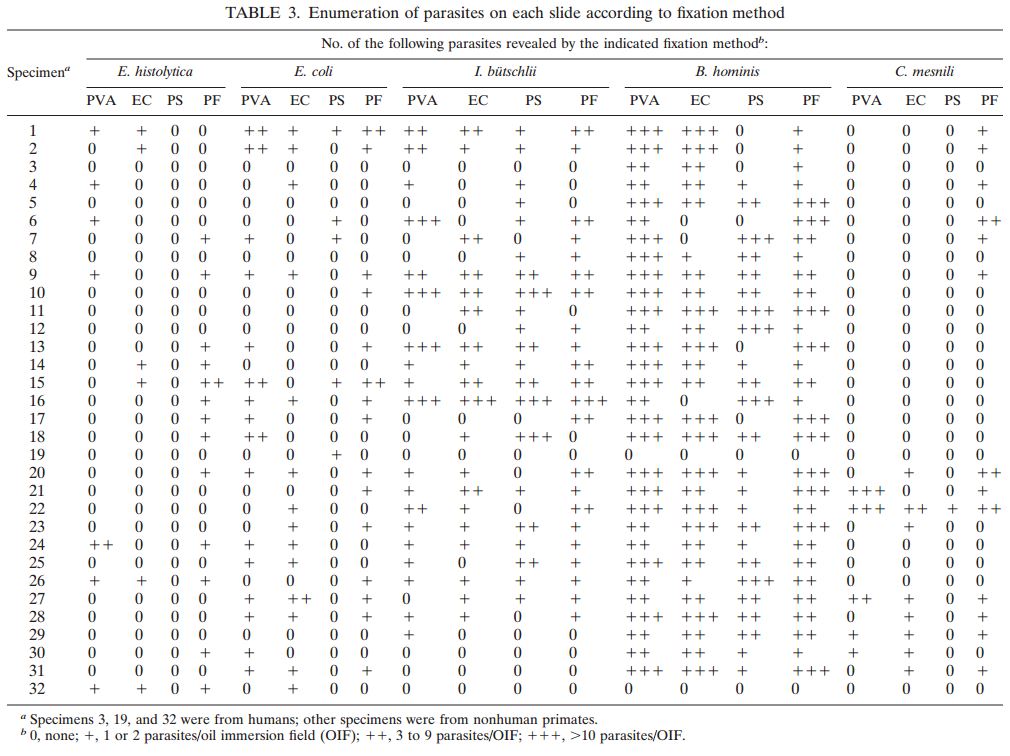
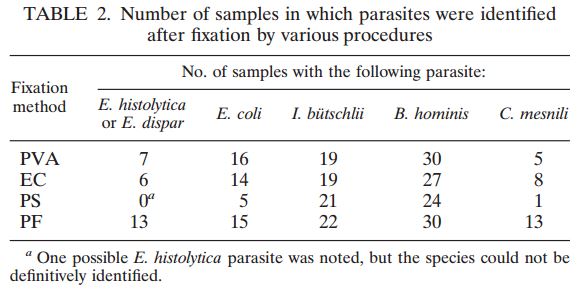
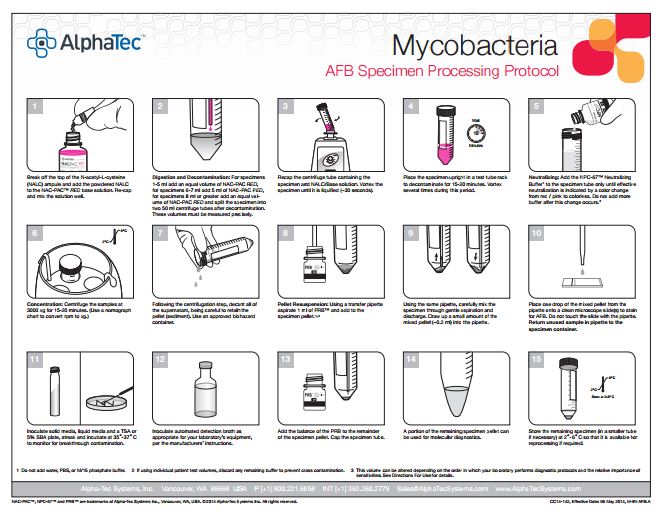
113 pulmonary samples were collected for the diagnosis of tuberculosis or other mycobacteria by the State Agency for Tuberculosis and Lung Diseases in Riga Latvia. Each sample was split into two aliquots of equal volume. Once divided, each sample was prepared for diagnostics using a separate pre-analytical methodology; the first being the conventional sodium hydroxide, tri-sodium citrate, N-acetyl-L-cysteine digestion and decontamination methodology per Kubica et al, the second using NAC-PAC™ RED, NPC-67™ Neutralizing Buffer, and PRB™ (Pellet Resuspension Buffer) from Alpha-Tec Systems, Inc.2 Each preparation methodology was functionally similar, requiring the addition of a digestion and decontamination agent, a 15 minute period of exposure, the addition of a buffer to dilute or neutralize the sample, centrifugal sedimentation at 3000xg, complete decantation of the resultant supernatant, and reconstitution with a small volume of buffer or diluent prior to diagnostic protocols. Once prepared for diagnostics, each aliquot was examined microscopically for the presence of acid fast bacilli using Auramine O & Rhodamine B staining and 100uL of each sample was cultured on Lowenstein Jensen media. Those samples that contained microscopically observable mycobacteria were assigned a concentration rating according to the WHO/IUATLD evaluation scale. 3 Those samples that showed mycobacteria growth in culture were scored based upon the CFU present.
Results
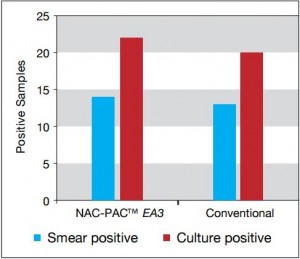
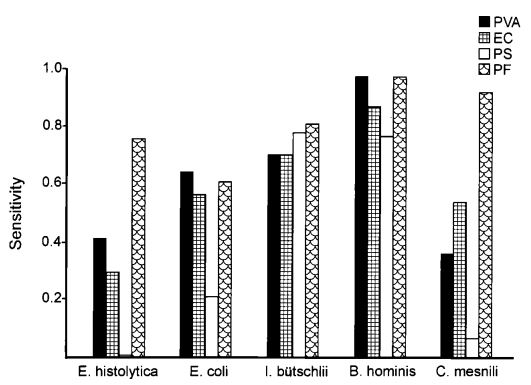
Figure 2: Comparison of smear and
culture positivity
Of the 113 samples submitted for diagnostics, 22 were positive for the presence of mycobacteria by either acid fast microscopy or culture on Lowenstein Jensen. Of these 22 positive samples, 22 were detected in samples prepared using Alpha-Tec reagents and 20 were detected using the conventional specimen preparation methodology. Both the conventional preparation methodology and the Alpha-Tec NAC-PAC EA3 yielded 13 smear positive samples with identical WHO/IUATLD scores, but 1 additional sample was smear positive when prepared using Alpha-Tec reagents that was not detectable until it grew in culture using the conventional methodology. Six of the samples were smear negative but culture positive using both methods; of these 6 samples, 2 showed equivalent growth, 3 exhibited greater organism recovery using the Alpha-Tec methodology and 1 exhibited greater recovery using the conventional methodology. Two samples were both smear and culture negative using the conventional specimen preparation method, but showed growth in culture using Alpha-Tec specimen preparation reagents.
Discussion and Conclusions
Tuberculosis is one of the gravest threats to public health the world faces today. With the rise of the AIDS epidemic and the emergence of drug resistant strains, tuberculosis is now a global health crisis, but despite this, diagnostic technology has remained essentially unchanged for decades. The vast majority of laboratories worldwide still rely upon acid fast microscopy which is notoriously inaccurate, and growth in culture which is susceptible to contamination and takes weeks to produce results. The limitations inherent in these diagnostic methods can have serious consequences for patients whose diagnoses are delayed or who are misdiagnosed entirely. The pre-analytical specimen preparation process for tuberculosis diagnostics is rarely given any consideration by the clinical laboratory, and in most respects it seems wholly disconnected from the diagnostic outcome. As it is performed by the majority of clinical laboratories, the specimen preparation process does indeed allow for the recovery of mycobacteria from the patient specimen. Once the specimen preparation protocol is examined, it becomes apparent that there are shortcomings which, if addressed, could allow for the recovery of a greater number of viable mycobacteria improving the accuracy of both culture and microscopy. The results of a comparison of the conventional protocol for preparing specimens for mycobacteria diagnostics and that provided by the Alpha-Tec NAC-PAC EA3 suggest that a more rigorous control of sample pH and processing methodology can have a meaningful impact on the final diagnostic outcome using both microscopy and culture techniques.
The Alpha-Tec NAC-PAC EA3 Specimen Preparation Reagent System:
- Identified mycobacteria infection that was otherwise undetected using conventional specimen preparation methodologies.
- Showed greater average recovery of mycobacteria in culture.
- Allowed for microscopic detection of samples that otherwise would not be identified.
References
1. Kent and Kubica, 1985. Public Health Mycobacteriology: A Guide for the Level III Laboratory. USDHHHS. Centers for Disease Control, Atlanta, USA. 2. Alpha-Tec Systems, Inc. NAC-PAC EA3 A.F.B. Digestion System. Package Insert Revision 2.00, Effective Date: 12/14/07. http://www.alphatecsystems.com/index.htm 3. World Health Organization. Laboratory Services in Tuberculosis Control. WHO/TB/98.258. Geneva, Switzerland: World Health Organization; 1998.
NAC-PAC™, NPC-67™ and PRB™ are trademarks of Alpha-Tec Systems, Inc., Vancouver, WA, USA. CC14-258, Effective Date: 3 Oct 2014, M-PO-LAT3.B ©2014, Alpha-Tec Systems, Inc. All rights reserved.